After the discovery of electrons, J. J. Thomson put forwarded the atomic model to describe the occurrence of electrons inside the atom. He considered electrons are scattered inside the atom as seeds are scattered inside the watermelon. Electrons are negatively charged particles. The positive charge diffused on the atom neutralizes the negative charges of electrons. That's why it is called the watermelon model of the atom.
Wednesday, 1 September 2021
Monday, 24 May 2021
Sigma and Pi bonds
sigma and pi bonds are two types of covalent bond. They differ each other on the mode of overlapping of atomic orbitals - head to head overlapping or side wise overlapping. First kind of overlapping gives sigma bond while second kind of overlapping gives pi bond.
Electron density concentrates between two nuclei. It can shield internuclear repulsion effectively. Then there is greater extent of overlapping of atomic orbitals. That makes sigma bond stronger.
Where as electron concentrates above and below the internuclear axis. So, it can not shield internuclear repulsion effectively. As a result there is lesser extent of overlapping on pi bond which makes pi bond weaker than sigma bond
Click here to watch video of sigma bond and pi bond.
Tuesday, 18 May 2021
memory trick for atomic weight
Here are some technique to memorize name of elements and atomic weight of elements. It is specially concerned for the elements having atomic number between 20 to 30. First of all, lets discuss how to remember name and symbol of elements from atomic number 21 to 30.
सचिन,तेन्दुल्कर,बिराट कोली,क्रिश गेल,मे , फिर,कोही,नयाँ, सेन्चुरी, जमाएगा
Now I'm going to tell you three rules to remember atomic weight. they are
i) 2n rule,
ii) (2n+1) rule and
iii) (2n +x) rule
2n and (2n+1) rule
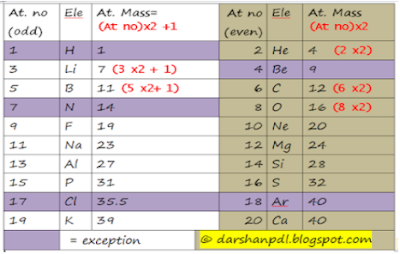
First two rules are applicable for the elements having atomic number up to 20. 2n rule is applicable for even atomic number and (2n+1) rule is applicable for odd atomic number. However, Be follows (2n+1) rule, nitrogen follows 2n rule regardless the atomic no. Atomic weight of Cl is 35.5 and that of Ar is 40.
even atomic no = 2n rule
odd atomic no = 2n +1 rule
Exceptions : H, Be, N, Cl & Ar
(2n+x) rule
It is applicable to memorize atomic weight of elements from Sc to Zn. It is easiest trick that gives whole number values only. where,
Atomic wt = 2n + x
where, n = atomic number of element and
x = Three वटा 45 three 55
That is, x = 3, 4,5,4,5,4,5, 3,5,5
Common names of organic compounds
Though IUPAC name is more systematic and unambiguous, common name or trivial name is widely used on organic chemistry as well as at the industries. vinyl chloride, ethyl chloride, benzyl chloride, benzal dichloride, benzotrichloride, benzoyl chloride etc are some common names.
Click here: list of common name of organic compound
ore and mineral
Different compounds- ores and minerals are more pronounced by their common names rather than IUPAC name. NaCl (Sodium chloride) is commonly called rock salt , table salt as well as common salt. Zinc carbonate is called calamine.
Click here: list of ore and mineral with their common name.
Thomson's atomic model
After the discovery of electrons, J. J. Thomson put forwarded the atomic model to describe the occurrence of electrons inside the atom. He ...

-
Here are some technique to memorize name of elements and atomic weight of elements. It is specially concerned for the elements having atom...
-
Different compounds- ores and minerals are more pronounced by their common names rather than IUPAC name. NaCl (Sodium chloride) is commonly...
-
sigma and pi bonds are two types of covalent bond. They differ each other on the mode of overlapping of atomic orbitals - head to head ov...