Here are some technique to memorize name of elements and atomic weight of elements. It is specially concerned for the elements having atomic number between 20 to 30. First of all, lets discuss how to remember name and symbol of elements from atomic number 21 to 30.
सचिन,तेन्दुल्कर,बिराट कोली,क्रिश गेल,मे , फिर,कोही,नयाँ, सेन्चुरी, जमाएगा
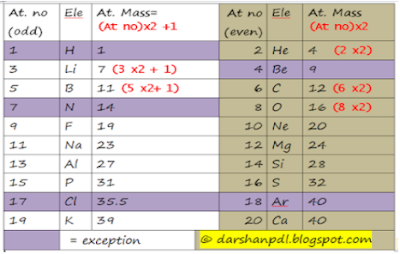
Now I'm going to tell you three rules to remember atomic weight. they are
i) 2n rule,
ii) (2n+1) rule and
iii) (2n +x) rule
2n and (2n+1) rule
First two rules are applicable for the elements having atomic number up to 20. 2n rule is applicable for even atomic number and (2n+1) rule is applicable for odd atomic number. However, Be follows (2n+1) rule, nitrogen follows 2n rule regardless the atomic no. Atomic weight of Cl is 35.5 and that of Ar is 40.
even atomic no = 2n rule
odd atomic no = 2n +1 rule
Exceptions : H, Be, N, Cl & Ar
(2n+x) rule
It is applicable to memorize atomic weight of elements from Sc to Zn. It is easiest trick that gives whole number values only. where,
Atomic wt = 2n + x
where, n = atomic number of element and
x = Three वटा 45 three 55
That is, x = 3, 4,5,4,5,4,5, 3,5,5





No comments:
Post a Comment